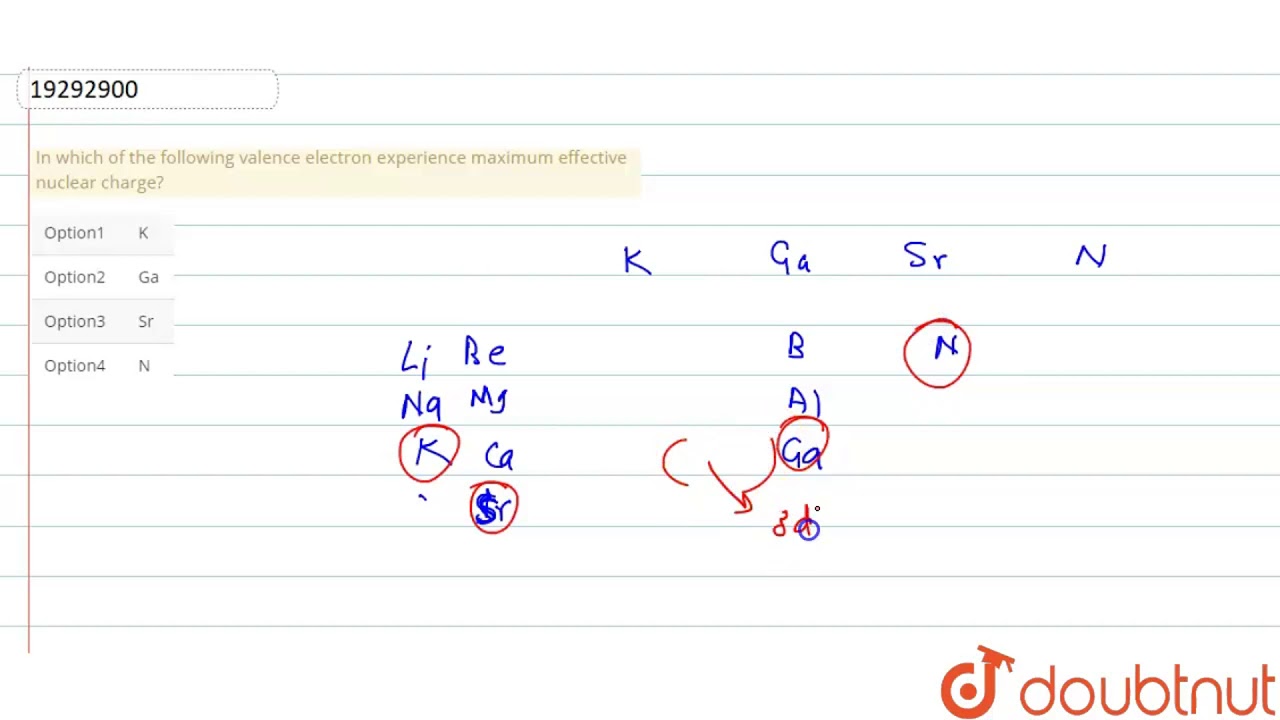
 Nuclear charge actually experienced by an electron is termed as effective nuclear charge The effective nuclear `Z^(**)` actually depends on type of shell and orbital in which electron is actually present. The relative extent to which the various orbitals penetrate is .`s gt p gt d gt f` (for the same value of n) The phenomenon in which penulitmate shell electrons act as screen or shield in between nucleus adn valence shell electrons and there by reducing nuclear charge is known as sheilding effect. The penultimate shell electrons repel the valence shell electron to keep them loosely held with nucleus . It is thus evident that more is the shielding effect, lesser is the effective nuclear charge and lesser is the ionizatio energy. In which of the following valence electron experience maximum effective nuclear charge?
Nuclear charge actually experienced by an electron is termed as effective nuclear charge The effective nuclear `Z^(**)` actually depends on type of shell and orbital in which electron is actually present. The relative extent to which the various orbitals penetrate is .`s gt p gt d gt f` (for the same value of n) The phenomenon in which penulitmate shell electrons act as screen or shield in between nucleus adn valence shell electrons and there by reducing nuclear charge is known as sheilding effect. The penultimate shell electrons repel the valence shell electron to keep them loosely held with nucleus . It is thus evident that more is the shielding effect, lesser is the effective nuclear charge and lesser is the ionizatio energy. In which of the following valence electron experience maximum effective nuclear charge?
Advertisement
Nuclear charge actually experienced by an electron is termed as effective nuclear charge The effect
Fiser
February 18, 2020
 Nuclear charge actually experienced by an electron is termed as effective nuclear charge The effective nuclear `Z^(**)` actually depends on type of shell and orbital in which electron is actually present. The relative extent to which the various orbitals penetrate is .`s gt p gt d gt f` (for the same value of n) The phenomenon in which penulitmate shell electrons act as screen or shield in between nucleus adn valence shell electrons and there by reducing nuclear charge is known as sheilding effect. The penultimate shell electrons repel the valence shell electron to keep them loosely held with nucleus . It is thus evident that more is the shielding effect, lesser is the effective nuclear charge and lesser is the ionizatio energy. In which of the following valence electron experience maximum effective nuclear charge?
Nuclear charge actually experienced by an electron is termed as effective nuclear charge The effective nuclear `Z^(**)` actually depends on type of shell and orbital in which electron is actually present. The relative extent to which the various orbitals penetrate is .`s gt p gt d gt f` (for the same value of n) The phenomenon in which penulitmate shell electrons act as screen or shield in between nucleus adn valence shell electrons and there by reducing nuclear charge is known as sheilding effect. The penultimate shell electrons repel the valence shell electron to keep them loosely held with nucleus . It is thus evident that more is the shielding effect, lesser is the effective nuclear charge and lesser is the ionizatio energy. In which of the following valence electron experience maximum effective nuclear charge?
Popular Videos
ONCE SHE HITS LATE GAME SHE'S UNSTOPPABLE! - Smite
December 05, 2019
THE NEW MOVEMENT TO REGULATE THE FUNERAL OF HINDU'S
February 16, 2020
How To Go From NOOB To PRO With NO ROBUX Roblox Adopt Me
January 07, 2020
8 Toxic Things Parents Say To their Children
December 21, 2019
Who the F*ck built that Toilet Door lock at my hotel?
January 07, 2020
PRECEDENCE AND ASSOCIATIVITY OF OPERATORS IN C|PROGRAMMING IN C-14
December 14, 2019
CHEETO CHRIST STUPID-CZAR - Randy Rainbow Song Parody
September 04, 2019
Recent
6/recent/post-list
HOT
6/random/post-list


0 Comments